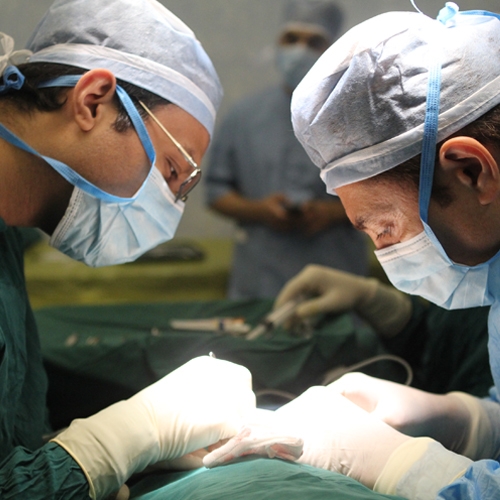
Production of Transplant Tissue Preservation Solution
 Iran FDA granted a production license for the first time to a transplanted tissue maintenance solution.
Iran FDA granted a production license for the first time to a transplanted tissue maintenance solution.According to the public relations and international affairs report of Royan Institute, The Transplanted Tissue Maintenance Solution (for organs such as kidneys and liver) was the first venture capital product of Royan Institute and Lotus Parsian Funding. After nearly five years of dedicated effort and follow-up, this product has finally obtained a 16-digit code IRC (Iran Registration Code) from Iran FDA. The IRC is an identifier and license for pharmaceutical products.
Nilfar Biotech company was in charge of the research and development stages of the WISCONSINIL brand product, and also Gene Asa Teb company, in cooperation with this company, was responsible for guiding the product until the final stage and its commercialization.
The commencement of the production of this medicinal product marks a significant milestone for the country. The hospitals and transplant centers in the country are in urgent need of at least 10,000 liters of this product per year. Presently, this medicinal product is only accessible through imports.
We anticipate that once the graft tissue preservation solution is domestically produced, its consumption will increase to 17,000 liters. It's important to mention that the local production of this product will result in a foreign exchange saving of over 5 million dollars.
" The image is decorative "
Publish date: 2024/03/11